Clinical Trials
Patient Engagement Technology with Unmatched Usability
Connectivity with digital support and trial management
Digitally enabled suite of solutions built on best-in-class and well adopted technology
Trial Experience Design
Optimize trial participation through an engagement platform configured around all aspects of each clinical trial.
Protocol Adherence
Trial management made for real life with a motivational experience informed by behavioral science, that keeps participants on track.
Interventions for Retention
Personalized, and proactive, interventions keep participants at ease, prepared and on-track throughout the duration of their trial.
Real-time Data Collection
Increase accuracy of electronic Patient Reported Outcomes (ePROs) with technology that promotes the longitudinal capture of data.
Advanced Engagement Technology
Empower participants in all aspects of their clinical trial journey with a hyper-personalized experience on a robust consumer-grade, patient engagement platform.
Keep participants connected with clinical operations and study coordinators throughout the trial with the right resources, at just the right time, to keep them confident and engaged.
Solution Suite & Supporting Technology
Digital Companion
A solution that drives engagement with a supportive experience by connecting participants with all trial resources.
Trial Management Connector
A web-accessible solution for all trial stakeholders - to actively monitor and interact with study participants.
Medisafe Maestro
Orchestrate an experience to precisely match study protocol and support the entire lifecycle of clinical trials.
Robust Data Analytics and Exports
Monitor patient generated data in real-time including ePRO/eCOA - and pull granular and unique data.
Deliver Higher Adherence, Persistence
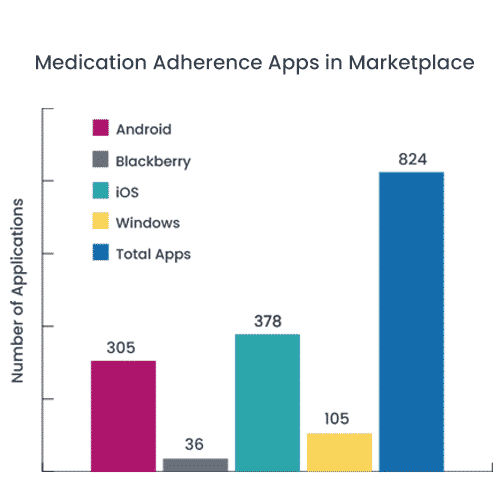
Medisafe ranked #1 out of 824 Medication Adherence Apps by the Journal of Medical Internet Research from both Health Professional and Consumer Vantage points. The aims of this study were to (1) provide an updated evaluation and comparison of medication adherence apps in the marketplace by assessing the features, functionality, and health literacy (HL) of the highest-ranking adherence apps and (2) indirectly measure the validity of our rating methodology by determining the relationship between our app evaluations and Web-based consumer ratings.