Access & Coordination
Transparency and Guidance in an Overwhelming Process
Integrated digital solutions that reduce the complexities of getting and paying for medications
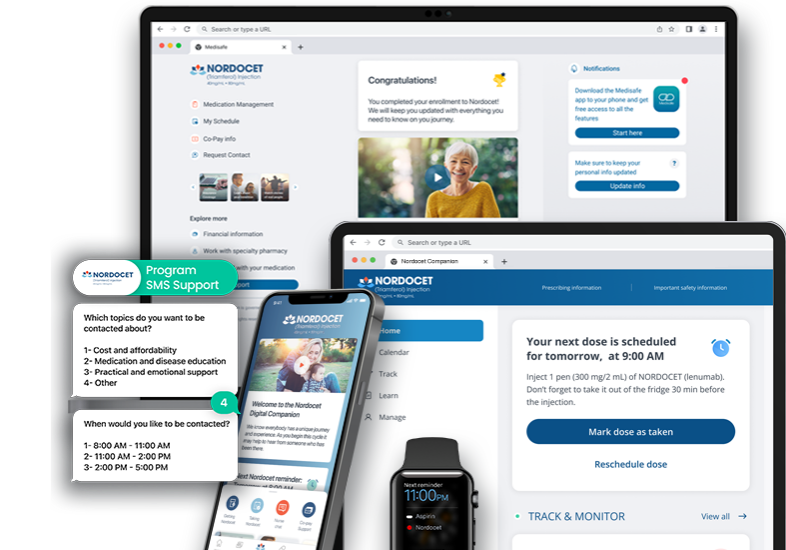
Seamless Enrollment
Automated invites drive program participation for better patient engagement.
Benefits Investigation
Actively engage patients in the eligibility process to expedite treatment start.
Prior Authorization
Integrated document exchange and status updates keep patients connected.
Shipment Tracking
Grant patients visibility into the shipment of their specialty medications.
Digital Document Exchange
Reduce arduous patient processes and increase care team efficiency and transparency with digital document submissions.
Extending Patient Support Programs
Medisafe’s digital companion is a single solution for patients as they start treatment.
Patients experience comprehensive support with contextualized educational, real-time status updates and streamlined routing of support. This includes everything patients need from personalized guidance and resources to when medication is prescribed, through benefits investigation, prior authorization and shipment tracking.
Technology & Innovation
Just-In-Time-Interventions (JITI)™
Precise and personalized advanced technology
Learn More
Medisafe Maestro
An integrated patient experience, reimagined
Learn More
Care Integration Engine
Technology that revolutionizes patient support
Learn More
Real-World Data Insights
Exclusive insights from real patient behaviors
Learn More
Proven Platform: Digital, Accessible Support
Medisafe’s integrated programs provide transparency to patients from prior authorization through first fill. In one case, leading to a 5% increase in first fill over for an existing support program.