Medisafe Presents Results of Study on Chronically Non-Adherent Patients
Over two-thirds of patients with hypertension, diabetes and depression became adherent after using Medisafe
NEW ORLEANS,LA – May 21, 2019. Medisafe, the personalized medication management platform with over five million users worldwide, announced today that further findings from its ongoing scientific study conducted by IQVIA will be presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 24th Annual International Meeting in New Orleans, LA. The meeting draws more than 4,000 health economics and outcomes research (HEOR) thought leaders and stakeholders from over 70 countries and is one of the world’s largest conferences for pharmacoeconomic and outcomes research.
The latest study findings showed clinically and statistically meaningful results across two standard healthcare industry adherence metrics:
- MPR, which tracks frequency of refill, where an MPR of 1.0 corresponds to a 100% timely refill rate; and
- Persistence, which measures duration of therapy (without refill gaps).
MPR Summary Results
Hypertension, diabetes and depression patients increased their mean MPR from below 0.6 before using Medisafe (“pre-app”) to above 0.9 after using Medisafe (“post-app”). Additionally, almost 70% of non-adherent patients (MPR <.8) pre-app became adherent post-app (See Figure 1).
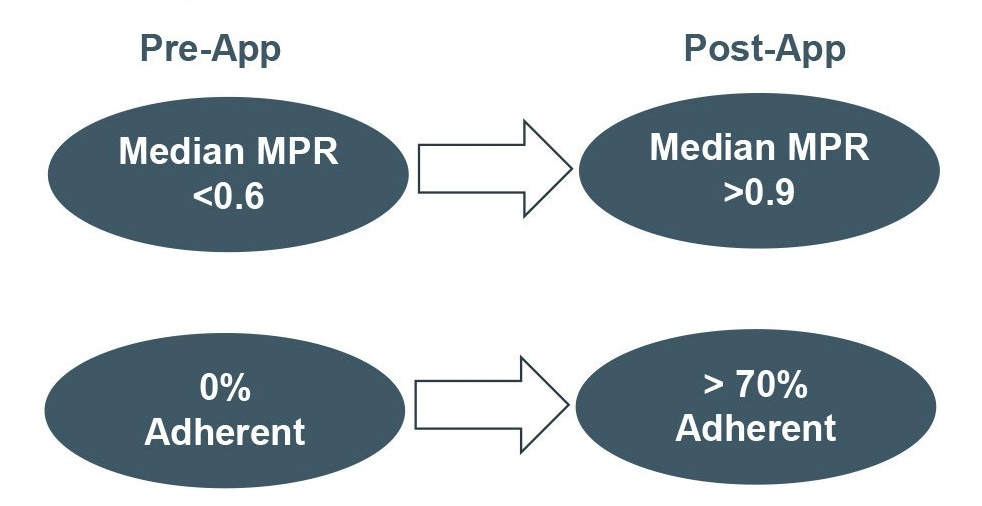
Figure 1
Persistence Summary Results
Twelve months after joining Medisafe, hypertension and depression patients were twice to three times as likely to be persistent than their matched control counterparts (Table 1). Over time, the difference in the therapy continuation rate between app users and controls continued to widen across all three therapeutic areas (Figure 2).
“By isolating and closely studying the cohort that was non-adherent pre-Medisafe, we now have our most promising evidence to date that Medisafe plays an instrumental role in getting chronic patients to refill their medications more often,” said Jon Michaeli, Executive Vice President at Medisafe and a contributing author of the research. “To improve outcomes, it is also critical to keep chronic patients on therapy longer, and we are pleased that Medisafe was able to demonstrate great success here as well.”
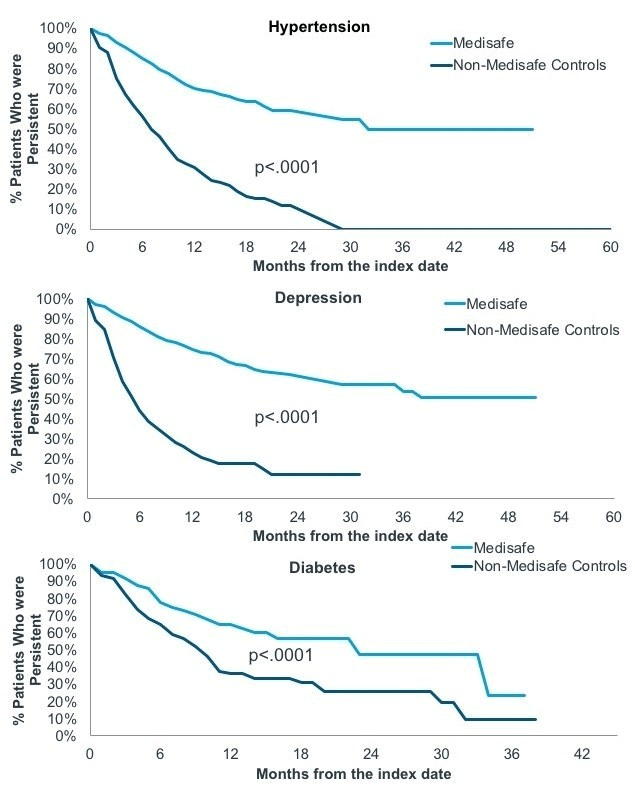
Figure 2
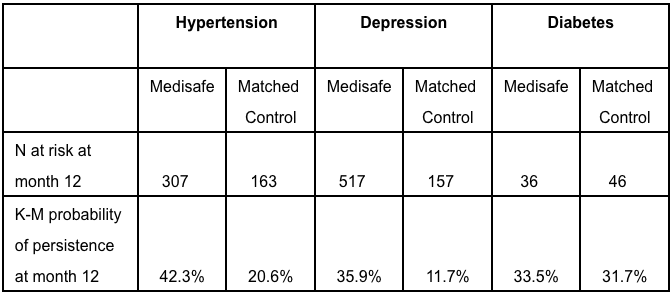
Table 1
Study Methodology
The study used retrospective patient data from Medisafe linked anonymously to IQVIA’s longitudinal prescription claims database (LRx). Users were selected based on the following criteria:
- Taking medications for hypertension, depression or Type 2 diabetes
- Joined Medisafe between 1/1/2014 and 12/31/2017
- Had a pre-app MPR <0.8
- Had at least 2 refills pre-Medisafe and 3 refills post-Medisafe
To calculate changes in refill frequency, patient pre-app MPRs were compared to their post-app MPRs. To calculate differences in therapy duration, Medisafe app user persistence was compared to that of non-app users (controls) based on comparable points in time in their treatment. App users were matched one to three to controls with pre-index MPR <0.8 in the same therapeutic area and claims in the same month as the app user.
A contributing author from IQVIA will present the study poster (PMU106) on May 21, 2019 from 6 to 7pm EDT during the conference poster session.
The study abstract is available on the ISPOR website and will be included in the ISPOR publication Value in Health, Volume 22, Issue S, 2019 May (Evaluating the Ability of a Mobile Medication App to Improve Adherence in a Chronically Non-Adherent Population in Hypertension, Diabetes and Depression, McGuiness CB, Michaeli J, Wang X, Wade RL).
