New Analysis Supports Medisafe’s Impact on Medication Persistence, Adherence
An analysis from Komodo’s Healthcare Map supports evidence of added value of Medisafe’s digital drug companion for specialty pharma programs
See the study here
BOSTON, June 30, 2021 — Medisafe, a leading digital drug companion company, today announced the findings from two validation studies examining the impact of its digital drug companion on persistence to specialty medications (Rx). Led by Komodo Health, the analysis used real-world patient data to examine the impact of digital drug companions to influence patient engagement. 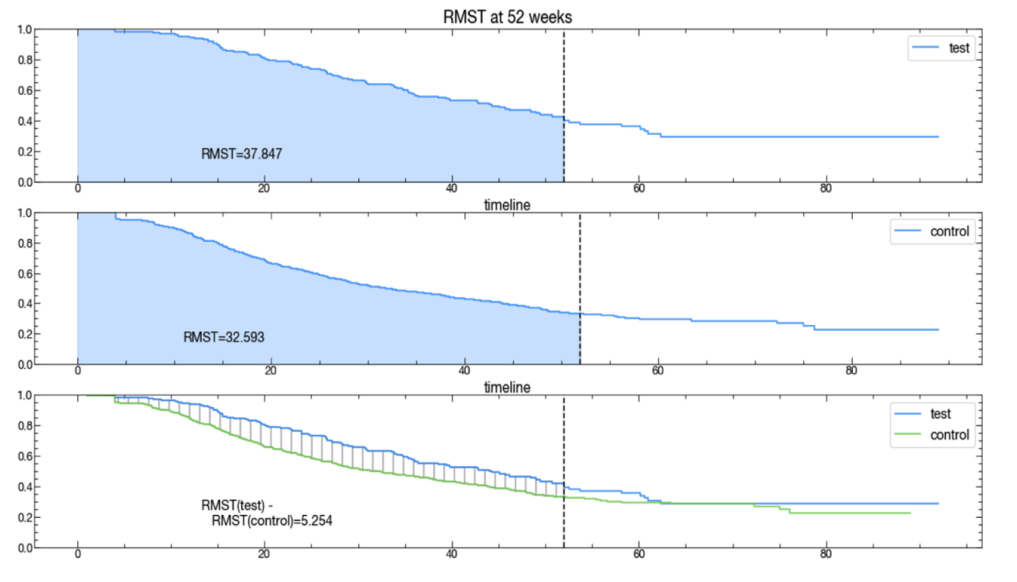 The finding indicates that specialty medicine patients extended persistence by 15%, and improved adherence by 7% among patients pre- vs.post Medisafe start.
The finding indicates that specialty medicine patients extended persistence by 15%, and improved adherence by 7% among patients pre- vs.post Medisafe start.
Findings underscore Medisafe’s role to help increase medication adherence and extend medication persistence among patients who used Medisafe’s digital drug companion, specifically designed to support their therapy. Results showed that existing patients who joined the Medisafe program saw a 7% increase in adherence pre- vs. post-Medisafe start compared to those not on Medisafe, who showed no statistically significant increase over the same time frame. In fact, Medisafe program users demonstrated a 5.8% adherence lift to 95.5% compared to the control group exhibiting a high adherence rate of 89.9% Patients using Medisafe remained compliant on the medication an additional five weeks compared to those patients not using a digital drug companion over a 52-week period.
“These impressive results demonstrate the types of measurable changes that are achievable when a digital drug companion complements medication therapy,” said Omri Shor, CEO and co-founder of Medisafe. “The findings represent real value that Medisafe brings to pharma organizations, underscoring the impact of patient-centric digital solutions on patients’ lives. When coupled with the financial costs of patient engagement, pharma’s investments into a digital drug companion can deliver quantifiable results that amount to significant financial value.”
Komodo Health compared Rx claims of patients enrolled in a biologic digital companion program against a matched control group. Medisafe worked with Komodo to identify test and control groups to create mirror cohorts. The key distinguishing factor of the test group was enrollment in the Medisafe biologic digital companion program. Medisafe users were required to have at least one relevant diagnosis, be active at least 90 days on the program, and be active on a biologic Rx while on the program. Additional matching criteria such as age, gender, and drug initiation date helped to ensure identical cohorts between test and control groups to produce accurate results.
“Real world evidence plays a crucial role in bringing the latest advances in science and technology to improve patient health,” said Arif Nathoo, MD, CEO and co-founder of Komodo Health. “This analysis highlights the important role of digital drug companions to support medication adherence, and Medisafe’s work with Komodo’s Healthcare Map enables deeper insight into the real-life healthcare experiences of very specific patient cohorts.”
While previous studies analyzed Medisafe’s ability to increase first-take doses among patients, the findings of the Komodo Health analysis show the impact of a digital drug companion to deliver specific therapeutic ongoing support for patients. The study was completed using de-identified patient data in compliance with applicable regulatory standards. Partnering with top global pharma companies and expanding opportunities across the health ecosystem, Medisafe’s digital drug companion supports more than 7MM patients throughout their unique therapy journey. To learn more about its advanced technology platform, visit www.medisafe.com.
