The Role of Digital Drug Companions in a Digital Therapeutics World
In the past eight weeks, the industry has been catapulted into a digital healthcare environment with regulations changing rapidly to support patients through social distancing times. The labeling of healthcare technology as digital health vs digital therapeutics stirs up the question, what qualifies as Digital Therapeutics?
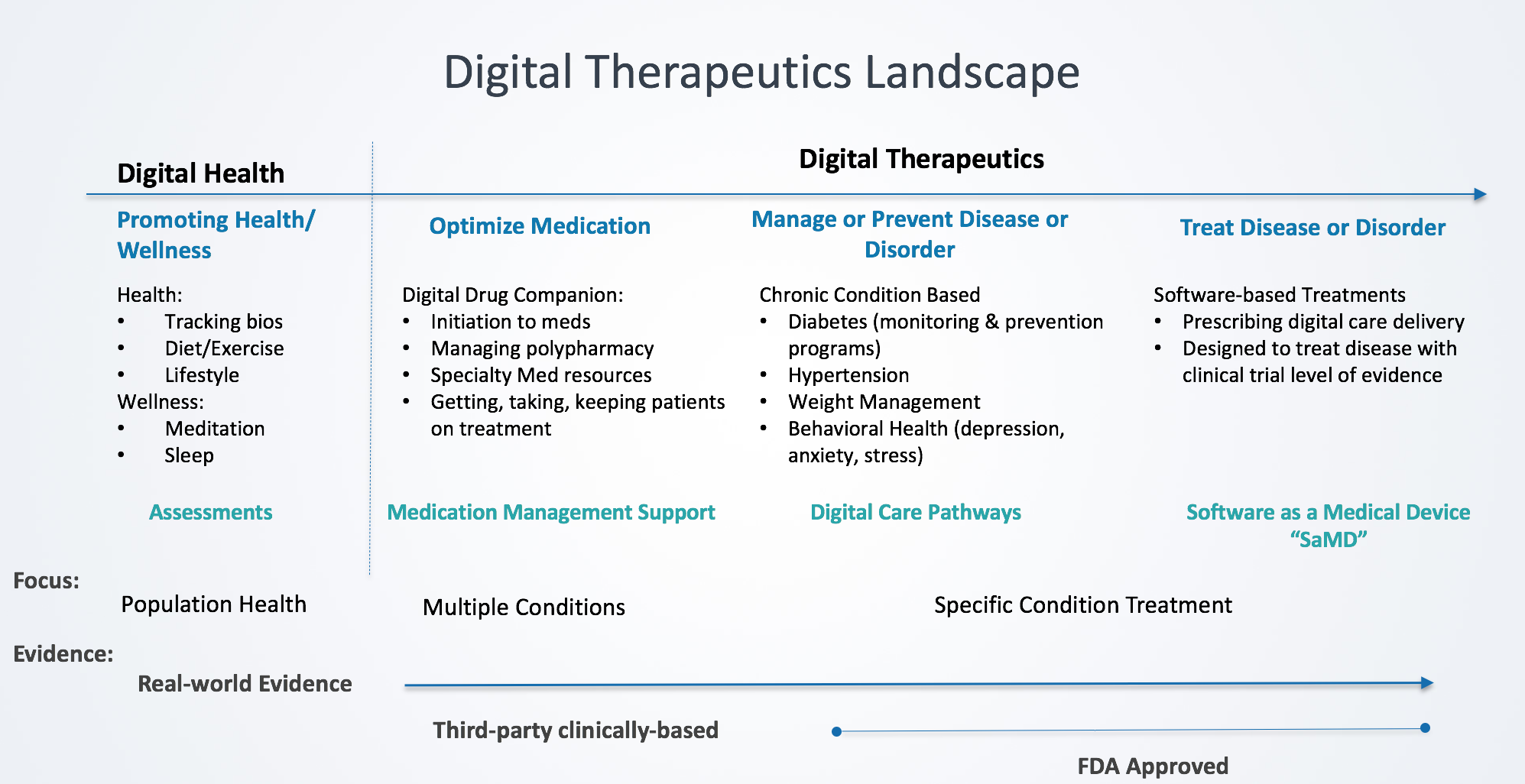
- Digital Therapeutics (DTx) are technologies that are clinically designed and validated in improving health outcomes
- Types of DTx are categorized by specific design characteristics that facilitate targeted outcomes including: Medication optimization, disease management and/or prevention, and disease treatment
- Digital Health is a term that applies to all technologies that engage patients around health and wellness
- The key differentiators between DTx and Digital Health are the specificity of therapy application and in methodology of results measurements
- The future of DTx is revolutionizing patient engagement and improving health outcomes IF the technology is easily adopted by consumers.
What are Digital Therapeutics?
According to Digital Therapeutics Alliance, “Digital therapeutics (DTx) deliver evidence-based therapeutic interventions to patients that are driven by high quality software programs to prevent, manage, or treat a medical disorder or disease. They are used independently or together with medications, devices, or other therapies to optimize patient care and health outcomes.” Digital therapy can take the form of mobile interactions, wearables with sensors, digitally connected medical devices or even virtual reality to name a few. These programs, while digitally executed, are clinically designed and validated to improve health outcomes.
What sets DTx apart from digital health is the targeting of clinical outcomes (vs health engagement) that is evidenced-based, clinically validated and regulatory adherent digital interventions. DTx products address a medical condition, manage or prevent a medical disorder or disease, optimize medication (an individual medication or class of pharmaceuticals), and/or treat a medical disease or disorder.[1]
Despite defining the category by outcomes and evidence, the interpretations and application of digital therapeutics vs digital health are somewhat ambiguous. Does digital therapeutics only target a particular clinical outcome in line with the defined clinical indication and patient population using the regimented intervention? Can a digital therapeutic be designed for a broad based population or does it only qualify for a hyper-specific patient population? Is a digital therapeutic only technology driven interventions or can there be a human element involved as well to support care? Do all digital therapeutic companies need to be FDA approved?
As this nascent industry evolves with the technology advances, let’s look at a categorization of the DTx landscape to better understand digital:
Digital Health
Digital Health applies to all technologies that engage patients around health and wellness. According to the FDA, digital health includes mobile health (mHealth), telehealth (a.k.a. telemedicine), devices, sensors and wearables, health information technology (HIT), personalized medicine and digital therapeutics.[2] While many digital health companies address medical conditions, they do so across a broad population providing diagnostic assessments with health tracking mechanisms. One of the key distinguishing elements from digital therapeutics is digital health technologies do not align with a specific therapy and clinically-based and real-world evidence. Results and measurements are typically broader based rather than therapy designed.
Digital Therapeutics Categories:
As we see the rapid pace of advancements in medical technologies to prevent, identify and treat medication conditions, it comes with the onerous of empowering patients to become medically-informed in order to manage the increasingly complex regimens. The following digital therapeutic categories empower patients with their treatment needs:
Optimize Medication – “Digital Drug Companions”
Digital drug companions support the patients navigate medications through their treatment journey. These digital companions guide patients from their medication initiation into designed programs supporting medication management. From the point that a patient receives their script from the provider, 30% of scripts remain unfilled. Patients require guidance to navigate challenges in medication access, especially for specialty medications, which require prior auth and coordination between Specialty Pharmacies, Hubs and payers. Digital drug companions expedite the path for patients to get their first fill and ultimately their first take through tracking the process and exposing patients to programs, such as financial assistance.
Digital drug companions have clinically backed evidence in increasing adherence and persistence on therapy. Think of the impact this has when managing critical medications warfarin, antiplatelets, insulin or antiretrovirals. One missed dose might have major impacts on overall health outcomes. Reasons for skipping are vast (from fear of injections to affordability of medication) and patients left without guidance and resources make potentially life threatening decisions to take a drug holiday. Digital interventions need to be timely and personalized to make it relevant in the patient’s journey with needed resources that expand this digital support beyond a “pill reminder”.
Manage or Prevent Disease/Disorder – “Digital Care Pathways”
Personalized digital guidance supports patients with chronic conditions to manage disease and prevent progression. With 147 million Americans living with one or more chronic condition, digital therapeutic programs guide patients through clinically-based care pathways to improve health outcomes. For example, these digital advancements motivate diabetes patients to manage their conditions by digitally connecting their blood glucose meter, test strips, timely expert support, and sending alerts all into a personalized program.
Software based selfcare tools also influence behavior change along with managing chronic conditions. Tracking behavior and care activities (such as therapeutic measurements) fuels the digital programs to dynamically provide personalized feedback keeping patients on care pathways. In addition to designing programs addressing clinical needs and outcomes, these digital care pathways incorporate behavioral psychologists to engage and influence patients along their journey.
Digital therapeutic solutions in this category do not necessarily require FDA approval but must have third-party validation of efficacy and safety claims on treatment. These solutions are not a replacement (adjunct) to existing therapy practices but enhancing the treatment plans (care pathways) through digital connectivity. Benefits to patients include digital guidance through complex conditions, support during times of uncertainty to keep patients motivated, and connectivity to experts, community and resources to stay on treatment.
Treat Disease or Disorder – “Software as a Medical Device” (SaMD)
When digital therapeutics emerged, this category “treating a disease or disorder” lead the evolution that a therapy could be delivered as a digital program. SaMD treatments in this category replaces traditional therapy practices through digital interventions such as digital gaming concepts, ingesting microchips that connect to bio sensors, and medical devices with digital connectivity for monitoring, to name a few. These adjunct (or alternative) therapies are prescribed to patients and monitored with clinical supervision. Examples of alternative therapies include replacing medication to manage depression or insomnia with sensory stimuli delivered via a mobile device. Conditions addressed by these digital therapeutics include cognitive impairment (ADHD, anxiety, depression), oncology, diabetes, asthma and addiction supervision. While these SaMD programs replace traditional therapies with digital engagement they collect and provide digital assessments, clinical evaluations and clinical supervision for patients as they manage side effects or track symptoms. These digital therapies are grounded in clinical evidence and meet approval standards of regulators.
In Summary
Medisafe’s digital drug companions support over six million users in medication management with clinically validated evidence of increasing adherence on average by 15-20%. The role of digital drug companions is to support patients improve outcomes of their medications but recognizing that patients today manage multiple medications or complex medication regimens, which becomes a daunting task. Holistically supporting patients requires support beyond a single condition approach but rather managing the patient’s entire medicine cabinet. In fact, on average Medisafe users under 65 manage five medications in their medicine cabinets and those over 65 that increases to seven. While DTx companions designed to treat specific conditions through care pathways or SaMD have demonstrated tremendous outcomes, we must also be aware of the rest of the story. According to the HHS, one in four Americans has multiple chronic conditions and that percentage goes up to three out of four in Americans over 65. Patients require digital hand-holding today more than ever to navigate multiple treatments. Digital therapeutics revolutionize the ability to empower patients and improve their outcomes if the technology is easily adopted by consumers. Understanding the needs of patients is critical and also incorporating the technology into everyday life is imperative to drive the desired engagement and support patients along their journey.
[1] Digital Therapeutics Alliance
[2] Dassault Systems: The Rise of Digital Therapeutics September 10, 2019
